
MDR vs MDD QUICK COMPARISON TSQuality.ch
Typically, all MDD-MDR transition projects initiate with Gap Assessment. Gap Assessment is a crucial activity during MDR transition & our team with engineering & regulatory expertise are well equipped to conduct this activity with an analytical mindset, resolve any regulatory concern and develop robust regulatory strategy for medical device.

MDR Guidance Medical Device Regulatory Guide
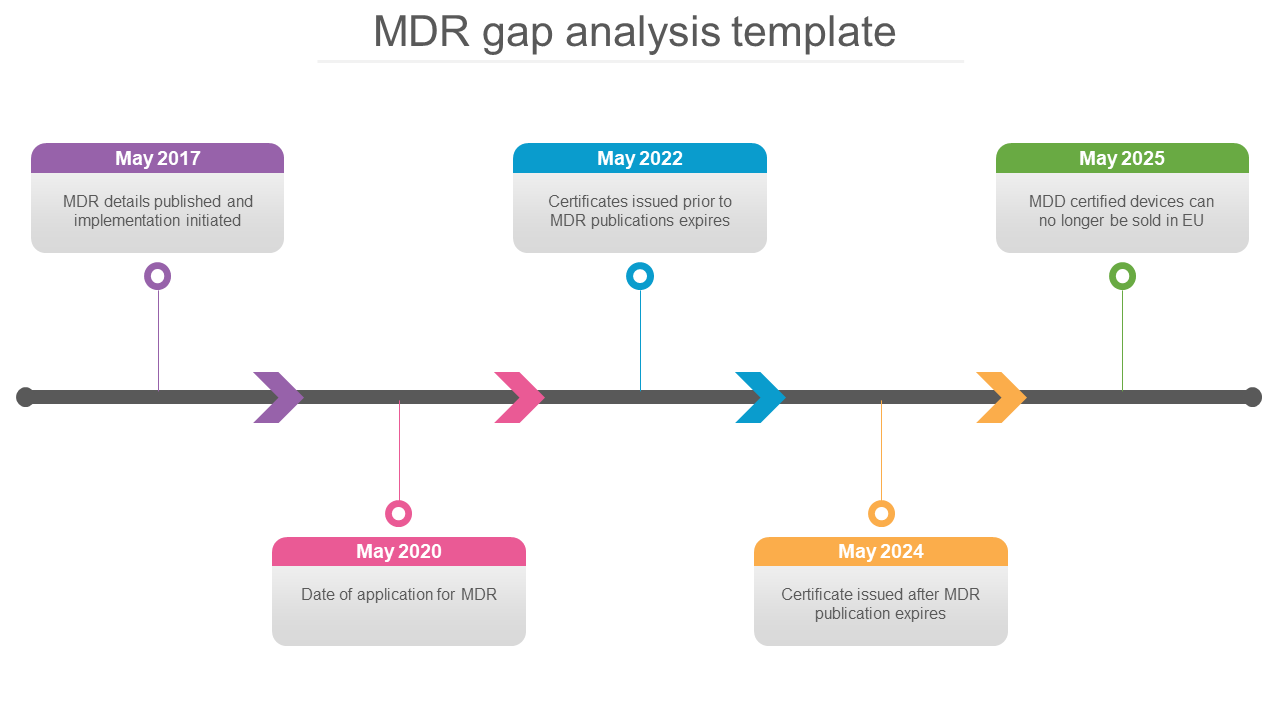
Practical approach for identifying Gaps between the MDD and the MDR September 2, 2020 Ossie Milanov, BA, Quality & Regulatory Project Manager What needs to be reviewed? Technical Documentation which must include: Device description and specification Information to be supplied by the manufacturer Design and manufacturing information

MDD to MDR Consultant for Medical Devices MDR Consultants IZiel
The MDR Gap-Analysis Tool supports medical device companies to implement the new medical device Regulation EU2017/745 in a easy way. The MDR Tool can be downloaded in English or German language. Furthermore also a Gap-Analysis of the new IVDR EU2017/746 is available and we are also offer Webinars and Consulting.
MDD vs. MDR Gap Assessment Tool Greenlight Guru
Home / Free MDR Gap Analysis Download our Free MDR Gap Analysis document This tool will help focusing the requirement introduced by the new MDR. You can download it free, fill it out and if you want send it back to us, so we can review it and help you plan the required activities. Your Name (required) Your Email (required) Company (required)
An MDR Gap Analysis Summary
6y Earlier today I have been searching for a Gap Assessment tool that compares the MDD to the MDR and allows companies to make that clause by clause analysis, I cant seem to find one, I did.
MDR Gap Analysis Template Slide
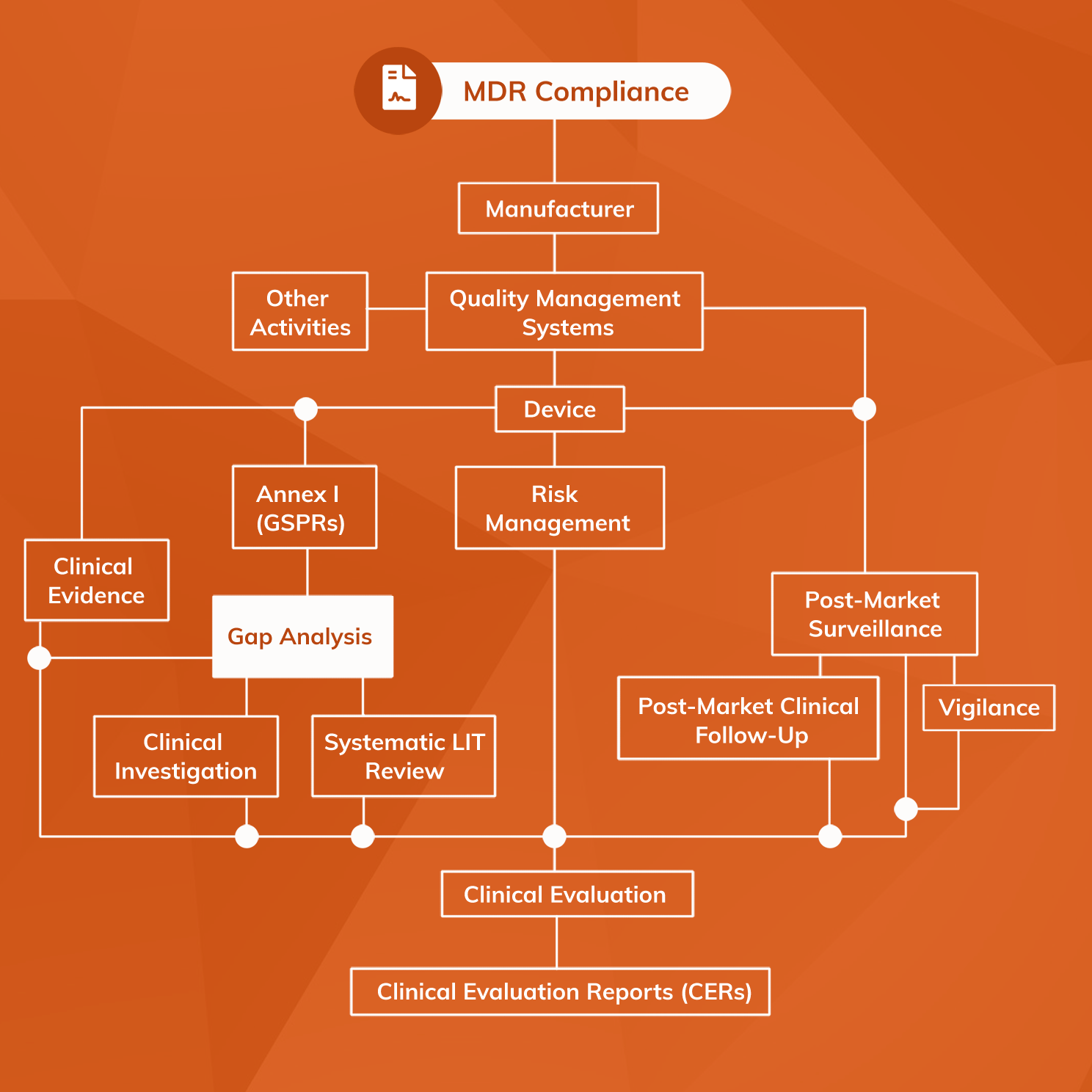
We Evaluate the Following as Part of Our EU MDD-to-MDR Gap Assessment: Current level of compliance with MDR General Safety and Performance Requirements Classification of current medical device (s) under the MDR Robustness of your CE technical documentation files, including links to risk management, postmarket, design, and other processes

MDD vs. MDR Gap Assessment Tool Free Download
MDR Technical File GAP analysis Checklist Based on the customer request, we have developed an open-source MDR Technical File Gap Analysis Checklist. This tool aims to assist regulatory professionals identify missing data/information in a ready to submit technical documentation file.

MDD vs MDR GapAssessment Tool
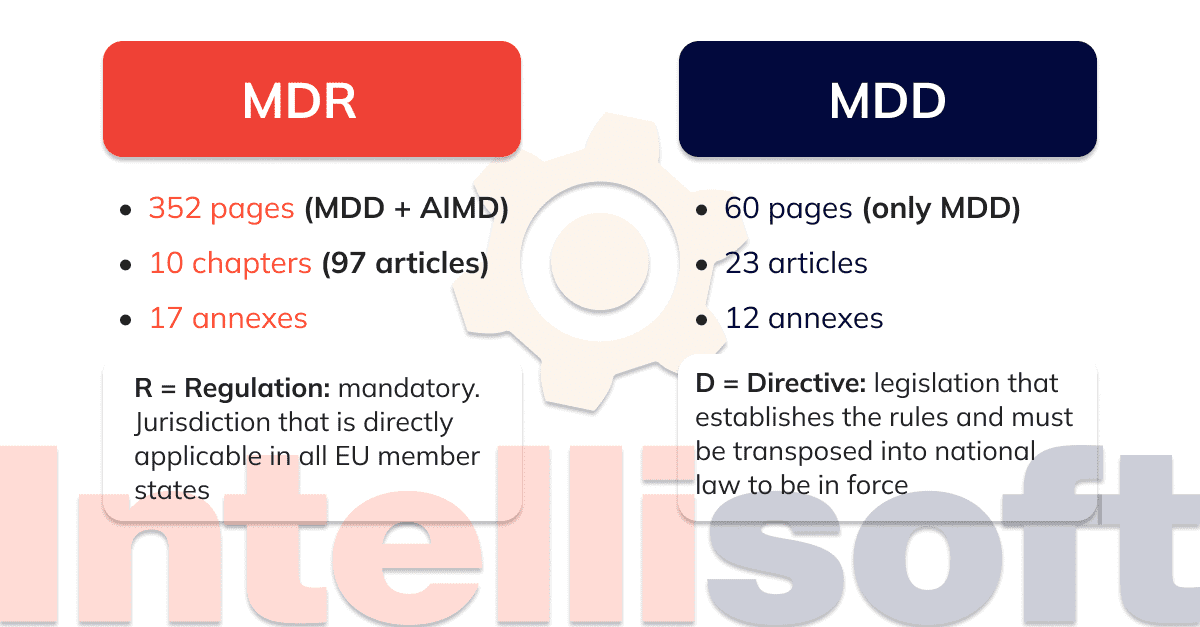
The Medical Device Directive - MDD - (Directive 93/42/EEC) was published in 1993. Its intention was: to harmonise the laws and standards relating to design and manufacturing medical devices within the European Union to ensure that medical devices are safe for patients

MDR vs. MDD 13 Key Changes
Whether a gap analysis between your current Medical Devices Directive (MDD) and MDR compliance is needed; Whether your current Technical Documentation can meet MDR requirements Learn how far your company has to go to reach MDR compliance for CE Mark certification with our Checklist.

MDR Compliance Gap Analysis Spreadsheet [format *.xlsx] M.L. Reo Consulting
Similarly, a gap analysis can be applied to your Quality Management System (QMS) mainly as there is now a strong link between QMS and the new Regulations. For more information, please get in touch with the BioReg team.

MDD to MDR Consultant CE Approval for Medical Device IZiel
The European Medical Devices Regulation 2017/745 (MDR) now applies in the world's second-largest medical device market. The new Regulation introduces major changes to how medical device manufacturers obtain CE Marking and maintain access to the European market, yet some companies may have yet to come fully into compliance with these new.

MDR vs MDD QUICK COMPARISON TSQuality.ch
An MDR Gap Analysis is the process of systematically examining a medical device's clinical evidence portfolio to determine whether it demonstrates conformity with all relevant MDR requirements. It is a crucial first step in developing and maintaining an MDR compliance strategy. Gap Analysis Tool Evaluation Checklist
EU Regulation Transitioning from the MDD to MDR
the many benefits of using this powerful tool in your transition process. 1:57. Greenlight Guru has teamed up with EU MDR expert firm, Regulatory Globe, to offer a free MDR Gap Analysis Tool to help companies with the transition process for compliance with new requirements for medical devices to be sold in the European market.

MDD vs. MDR Gap Assessment Tool Free Download
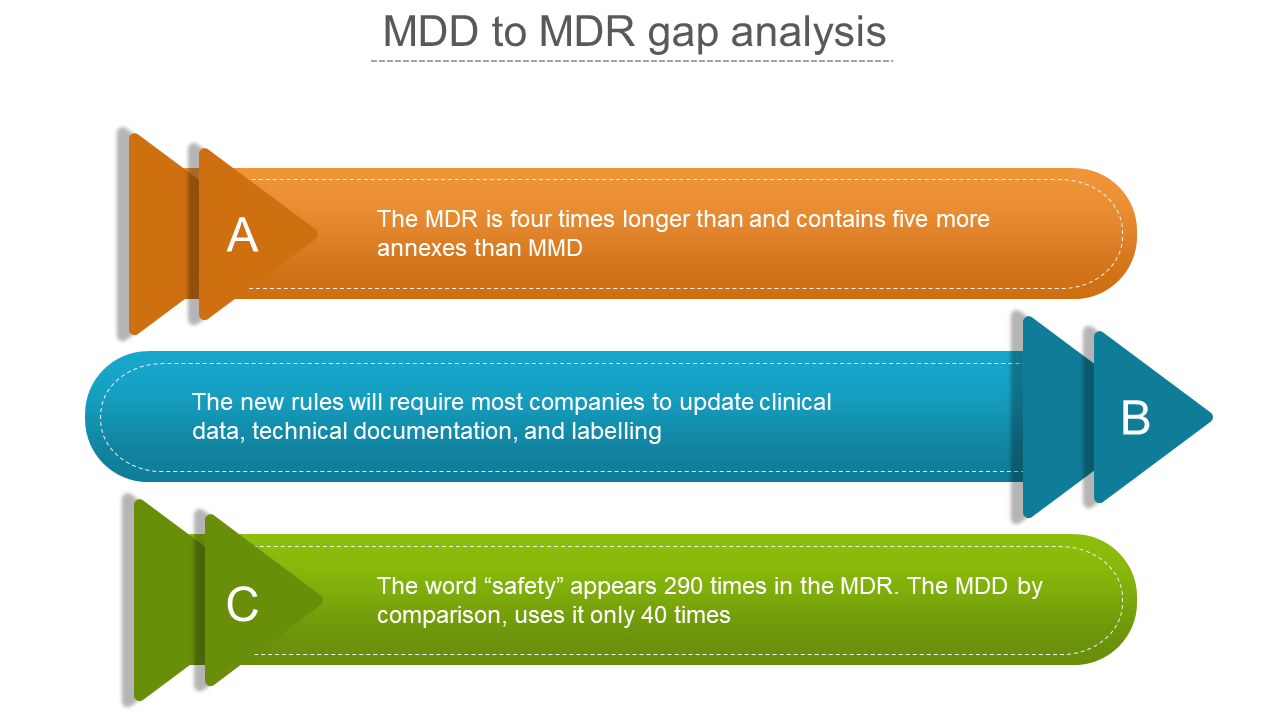
17 June 2022 MDR vs. MDD: 13 Key Changes ⚠️ Update: See below for important updates on EU-MDR compliance. If your time is short: Europe's new Medical Devices Regulation (MDR) will bring significant regulatory changes that may impact multiple business units within your organization.
MDD To MDR Gap Analysis Template PowerPoint & Google Slides
Hi Fellows, I just finished my first MDR gap assessment. Truly the best resource is BSI Transition to MDR page. Specifically, I recommend the following: 1. MDR Readiness Review - this is a nice sanity check for MDR readiness. 2. How to prepare for and implement the upcoming MDR - Dos and Don'ts - this has a more comprehensive review of each chapter in the MDR and what to pay attention to.
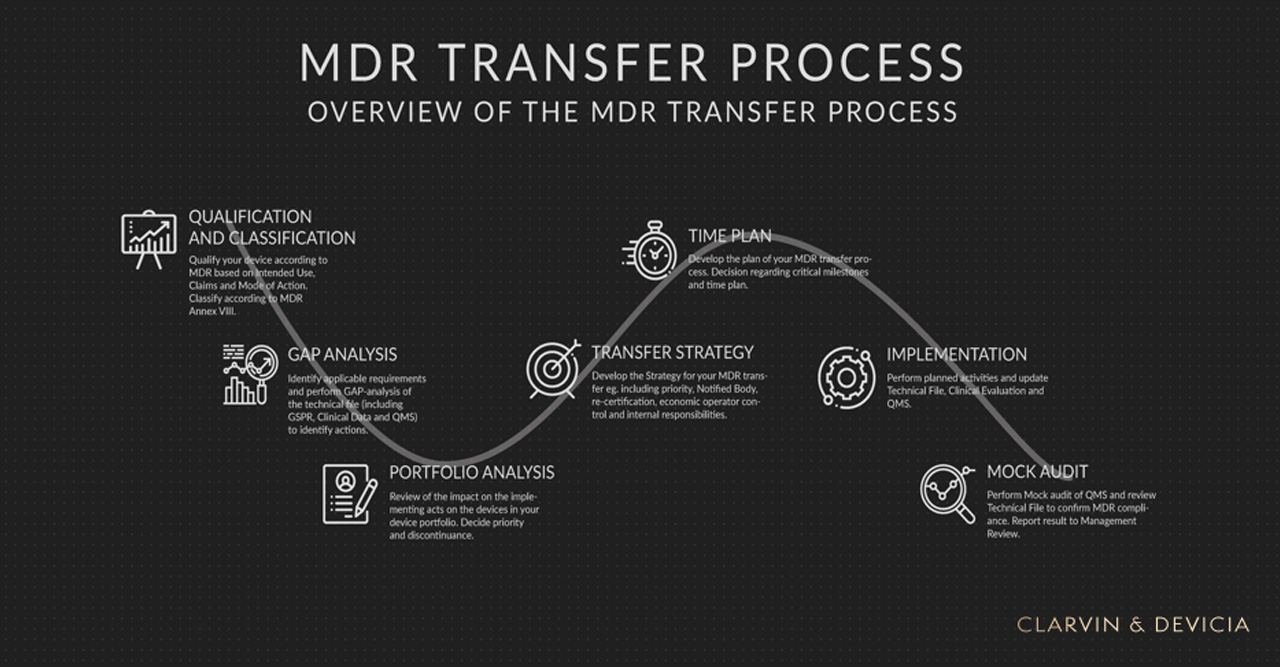
How to transfer from MDD to MDR? Devicia AB
In Annex I (1) of the MDD, the Directive clarified that design of devices shall include: "consideration of the technical knowledge, experience, education and training and where applicable the medical and physical conditions of intended users (design for lay, professional, disabled or other users)."
